Popular Shoulder Replacement Device Recalled Due To Alarmingly High-Rate of Failure & Complications...
What To Do If You Had Shoulder Replacement Surgery Between 2008 and September 2015...
From the desk of:
Andy Bederman, Managing Attorney
To Shoulder Replacement Patients:
If you (or a loved one) received a shoulder replacement between October 2008 and September 2015, this may be the most important message you should read...
Here is why...
On February 16, 2017 the FDA initiated a Class I Recall of a widely used shoulder replacement device called the Zimmer Biomet® Comprehensive Reverse Shoulder System™.
This device was recalled due to an alarmingly high-rate of failure and complications.
Please continue reading as these new developments may have a significant impact on you...
What Is The Zimmer Biomet® Comprehensive Reverse Shoulder System?
The Zimmer Biomet® Comprehensive Reverse Shoulder System™ is an artificial shoulder used in shoulder replacement surgery.
This reverse shoulder system was widely used with patients that had...
- Rotator cuff tears
- Shoulder arthritis (a.k.a. arthropathy)
- Previously failed shoulder joint replacements
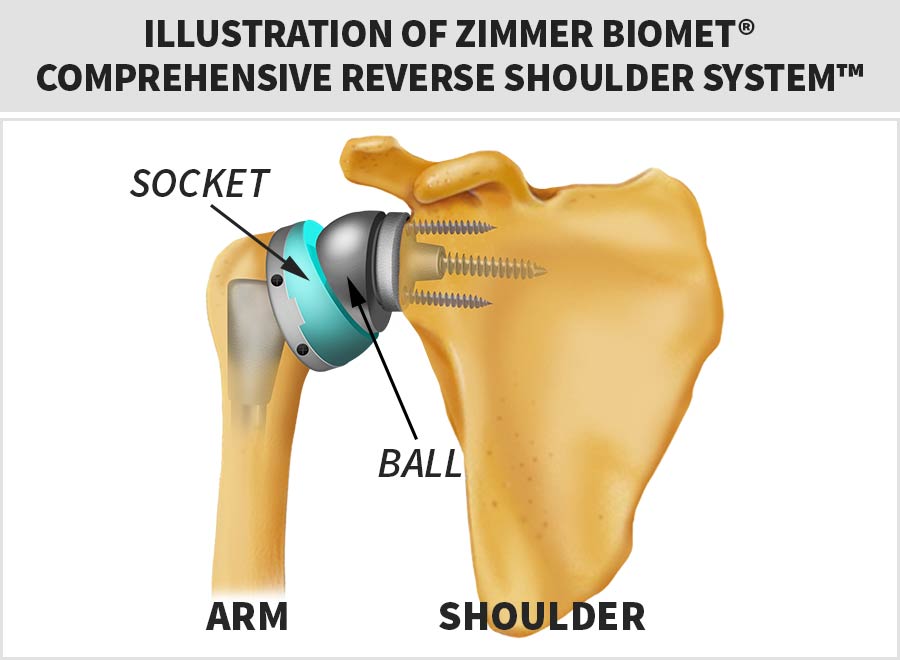
The Zimmer Biomet® Comprehensive Reverse Shoulder System™ was unique because it reversed the traditional placement of the “ball” and “socket” of the shoulder.
You will see in the diagram below, the device puts the “ball” on the shoulder (glenohumeral joint) and the “socket” on the end of the upper arm (humeral head), which is opposite to traditional total arthroplasty (shoulder replacement) systems.
Why Was This Shoulder Replacement Implant Recalled?
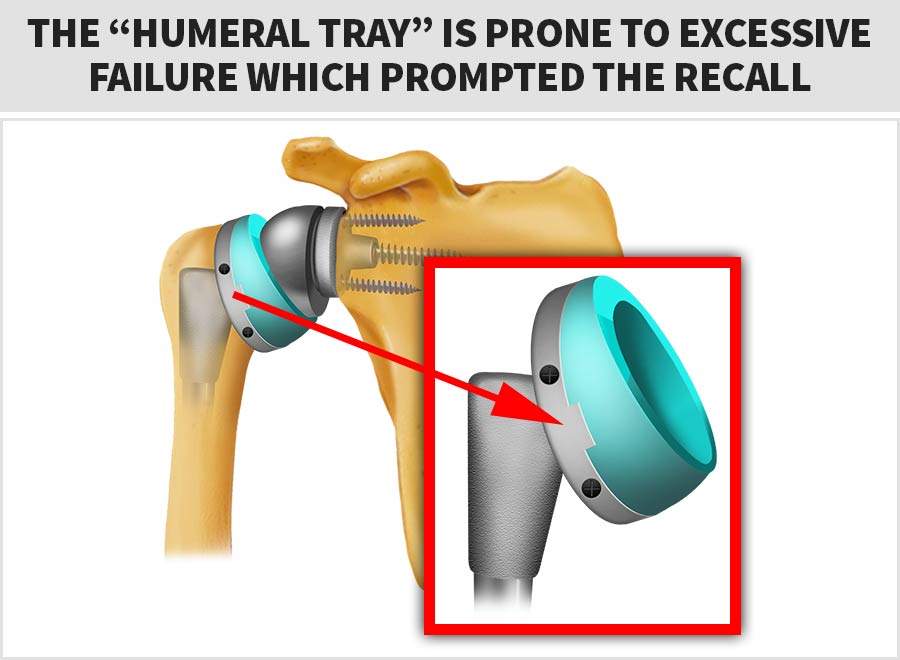
On Feb. 16th, 2017 the FDA initiated a Class 1 Recall of the Zimmer Biomet® Comprehensive Reverse Shoulder System™ due to an alarmingly high-rate of fractures with the humeral tray component.
The diagram below shows the device’s “humeral tray”, which is the part prone to excessive failure that prompted the FDA’s Class 1 (most serious) recall:
Patients who received this defective shoulder replacement device have been experiencing (or are prone to) the following complications:
- Device fracture or failure
- Loss of mobility or function
- Need for revision surgery
- Infection
- Pain
- And at worse, death
How Does This Recall Affect Me?
Please understand: The FDA issued a Class 1 recall for the Zimmer Biomet® Comprehensive Reverse Shoulder System™.
This is the MOST SERIOUS type of recall that the FDA can issue.
The FDA defines this Class 1 recall as:
“There is a reasonable probability that the use of a violative product will cause serious adverse health consequences or death.”
This recall is limited to Zimmer Biomet® Comprehensive Reverse Shoulder Systems™ that were used between October 2008 and September 2015... which encompasses approximately 3,650 systems used in this period.
Would I Have Been Notified If I Received The Zimmer Biomet® Reverse Shoulder System?
Patients who received the dangerous Zimmer Biomet® Comprehensive Reverse Shoulder System™ are likely NOT aware that they were given this device.
Sadly, there is currently no mandate for surgeons or health facilities to inform affected patients.
On December 20, 2016 Zimmer Biomet® sent an Urgent Medical Device Recall Notice to affected hospitals and surgeons who had used the recalled device.
This notice stated that there are no specific patient monitoring instructions related to this recall that are recommended beyond existing surgical follow up protocol.
Because of this, it is extremely important to take your health into your own hands – and it is well advised for all patients who received shoulder replacements from October 2008 to September 2015 to find out if they received the recalled Zimmer Biomet® Reverse Shoulder System™.
What This Means For You...
If you are still reading this, it means you likely fall into this scenario...
DOES THIS SCENARIO DESCRIBE YOU?
- I received an artificial shoulder replacement between October 2008 and September 2015 and have experienced loss of mobility, complications or require revision surgery.
Our law office is currently investigating these cases.
If you are unsure if you received the Zimmer Biomet® Reverse Shoulder System™, our law firm is helping people find out if they received the recalled device.
With your authorization, we are able to obtain your surgical records and confirm whether the recalled device was used in your shoulder replacement surgery.
I Experienced Complications That Required (Or Will Require) A Revision Surgery...
If you received the Zimmer Biomet® Reverse Shoulder System™, you may have experienced any of the following negative effects that will require or did require a revision surgery:
- Loss of mobility or function
- Severe Pain
- Device fracture or failure
- Infection
So please continue reading because it is critically important for you to understand not only the medical aspects of this, but also the legal aspects related to your safety and health.
This Is Not The First Zimmer Biomet® Shoulder System To Be Recalled
Zimmer Biomet®, the medical device manufacturer of the Comprehensive Reverse Shoulder System™ previously had to recall OTHER reverse shoulder devices used in surgeries because of product defects.
In September 2010, Biomet, Inc. recalled a Comprehensive Reverse Shoulder humeral tray due to high rates of failure.
In April 2011, Biomet, Inc. had to recall another Comprehensive Reverse Shoulder Humeral Tray because it could potentially contain a component that was incorrectly assembled.
This is not a great track record in terms of device safety -- calling into question the reasons for so many related recalls from this manufacturer...
Zimmer Biomet® Chose To Skip Rigorous Pre-Market Research That Could Have Uncovered The Serious Risks of the Reverse Shoulder System
Zimmer Biomet® (the manufacturer) chose to bring the Comprehensive Reverse Shoulder System® to market without the typical FDA-required pre-market research that gives doctors and patients confidence a medical product is safe to use.
Instead, Zimmer Biomet® sought FDA approval via the highly controversial 510(k) exemption process -- which allows "low risk" devices to bypass the rigorous pre-market research and trials.
However, the FDA's 510(k) exemption requires the Comprehensive Reverse Shoulder System® to offer a reasonable assurance of safety and effectiveness, and per the FDA's website...
"510(k) exempt devices must be suitable for their intended use"
Sadly, the FDA’s most serious Class 1 Recall shows that the Comprehensive Reverse Shoulder System® is not safe, and never was effective or suitable for its intended use.
You May Be Eligible For Financial Compensation Having Been Affected By A Defective Shoulder Replacement
Medical device manufacturers are subject to STRICT guidelines and rules by the FDA.
The FDA puts rules in place that require medical device manufacturers to conduct extensive testing, research and clinical trials before their device can be used in surgeries.
This is done so doctors and patients like yourself can weigh the benefits of using a particular medical device against the possible dangers and consequences it may pose.
And it gives doctors the ability to make an informed decision about which brand shoulder replacement system to use, based on available safety data gathered in pre-market testing and trials.
When A Manufacturer Doesn't Adequately Test The Safety of A Shoulder Replacement Implant, Doctors & Patients Become Victims -- And All You're Left With Is A Dangerous Device Stuck Inside You...
As a result of Zimmer Biomet® failing to design a sufficiently safe and effective shoulder replacement system, you or a loved one may have suffered negative health consequences, needed revision surgeries or therapy, experienced emotional pain, incurred lost wages due to time off work, or endured other significant expenses.
Because of this, you may be entitled to what are called "damages", which is just a legal term for money awarded in individual lawsuits, class action lawsuits and settlements.
Because the Zimmer Biomet® Comprehensive Reverse Shoulder System™ has such a high-rate of failure, complications and need for revision surgery, patients often find themselves burdened with substantial medical expenses and a reduction in their quality of life.
What Your Next Step Should Be If You Had A Shoulder Replacement Surgery Between 2008 and 2015...
Our legal team has reviewed the available evidence and research, and our experience in medical device liability cases tell us, that we are in prime position to help patients who are victims of the Zimmer Biomet® Comprehensive Reverse Shoulder System™.
We are offering a free legal consultation and case evaluation to patients who believe they may be affected.
If you'd like to learn more and speak with a defective shoulder replacement lawyer free of charge, this is your opportunity to discover the help that is available to you.
Getting started is simple and easy...
Fill out the contact form below or call us at 877-346-6177 for a free case evaluation and legal consultation. We will personally speak with you about your case.
Speaking to an attorney, you will get answers to the following...
- How do I find out if my surgeon used the Zimmer Biomet® Comprehensive Reverse Shoulder System™ in my shoulder replacement operation? (if you don't already know)
- Am I eligible to pursue a Zimmer Biomet® Comprehensive Reverse Shoulder System™ lawsuit or settlement?
- What is involved and how does it work?
- Should I file a lawsuit or seek a settlement? Individually or in a class action?
- Why this is not a class action, and why that is good news for you.
- How do I get started?
- ...And more
This consultation with an attorney is 100% free and confidential.
You will speak to our attorneys -- lawyers with years of experience getting successful legal outcomes for victims of defective medical devices.
We are currently accepting cases from all 50 states in the United States, so you are eligible to speak to us for free -- no matter what state you live in.
You should contact our firm if:
- You underwent a shoulder replacement surgery between October 2008 and September 2015 and subsequently experienced complications that required (or will require) revision surgery.
And know this...
If at any point you do not believe we are the most qualified to help you get the compensation you rightfully deserve, you are not obligated in any way, shape or form to pay us or work with us. This is a no-pressure consultation.
We are confident you will see the value we offer patients. And our law firm works strictly on a contingency basis -- meaning patients we represent only pay out of their recovery if they make a recovery in their case.
You must act quickly because there is only a small window of time that shoulder replacement patients can pursue financial compensation in the form of a settlement or lawsuit. This is called the "statute of limitations" -- and it is different for every state.
So act now and fill out the contact form at the bottom of the page. Or simply pick up the phone and call us right now at 877-346-6177.
Get the information you need to make the right decision for you.
Sincerely,
Andy E. Bederman
Managing Attorney
Law Offices of Greenberg & Bederman, LLC
Get Your FREE Legal Consultation!
Simply fill out the form below and one of our lawyers will promptly call you for your free legal consultation!
Want to call us instead? Call our attorneys at 877-346-6177 right now!
CONTACT US
Silver Spring, MD 20910
Local: 877-346-6177 (24/7)
Toll-Free: 877-346-6177 (24/7)
Email:
AWARDED SUPER LAWYER™ STATUS IN 2024
The Law Office of Greenberg & Bederman has numerous attorneys that were awarded the prestigious Super Lawyers™ status. Super Lawyers™ is an independent, attorney ranking organization that awards attorneys each year based on many factors, including affinity with other lawyers.
Copyright © 2024 Greenberg & Bederman, LLC.
All Rights Reserved.
Greenberg & Bederman, LLC is associated with Janet, Jenner & Suggs, LLC
Click to Scroll to Top of Page